By Dilish K Daniel
The government’s new draft policy reflects the two great themes it has been pushing forward since coming to power – ‘Ease of business’ and ‘Make in India’. The underlying theme of this draft prepared by the Department of Pharmaceuticals is very evident,’ One drug- One price’. Thus, all homogenous drugs will be priced alike removing the ‘brand effect’ on pricing. Currently, this draft is focusing primarily on the generic category of medicines. By its implementation, the government plans to fight unfair practices in the industry, cap trade margins, give boost to local manufacturing and make drugs cheaper.
The new policy proposes changes in both the role and function of the National Pharmaceutical Pricing Authority (NPPA) as well as its overall structure. NPPA was constituted in 1995 and is given wide-ranging powers to regulate the pharmaceutical industry in India. They are given the powers to fix maximum prices at which medicines and several medical devices can be sold to patients. NPPA also has the authority to cap prices of any medicine or medical equipment under extraordinary circumstances, in public interest. If the draft proposal is accepted, NPPA will lose its current autonomy. In its place, the Union government will have a greater role in deciding prices of medicines and medical devices.
PROPOSED NEW STRUCTURE FOR NPPA
Presently, NPPA functions as an independent body of experts, comprising of a Chairman (presently an IAS officer), independent member secretaries and an internal advisory team, largely sheltered from the Union Government’s influence. Under the new proposal they will have a three member team comprising of a Chairman, member (enforcement) and member (pricing) all of whom are to be selected by the government. NPPA can make decisions only after they get consensus agreement from all these three members. Additionally, it will have members with expertise in the field of pharmaceuticals, economics and cost accountancy. Government will also nominate an advisory body that includes members of civil society, doctors, pharmacists, industry representatives and government officials.
LEGAL FRAMEWORK
The Central Drugs Standard Control Organisation will be required to conduct annual audits of drug companies’ manufacturing facilities. It is mandated that Central and State drug regulators give clearances to manufacturers within a period of three months – extendable by another three months at best. The government will ensure that such clearances are given on a fast track basis. The draft also suggests that the manufacturers will be allowed to self-certify that their products are compliant with relevant tests. In a private-public partnership arrangement, pharmaceutical parks will be created at various places with an objective of bringing more ease of doing business.
UNIFORM PRICING
The new policy also states that ‘National List of Essential Medicines’ (NLEM) which recommends pricing caps mentions only the molecule name as against the practise of including details about strengths and dosages. The previous practise allowed pharmaceutical companies to make minor adjustments to the strengths of the drug and market it as a “new drug” outside price control. By stating only the molecule name, all strengths and dosages of the medicine will fall under the same price cap. In addition, the policy also seeks to bring down the unreasonable trade margins offered by various stockists to hospitals thereby bringing more standardisation to the industry.
OTHER SALIENT FEATURES
- Encourages indigenous manufacturing to reduce dependence on imports. As an incentive, locally manufactured drugs will be taken out of pricing controls for a stipulated time.
- Proposes to discontinue loan licensing or third-party manufacturing. Thus companies would be unable to outsource their production. They will have to use their own facilities. Previous cases of poor quality medicines from such outsourced facilities are the main reason cited for it.
- Ceiling price of regulated medicines to be linked with wholesale price index.
- The draft policy further recommends that the prices of drugs still under patents must be regulated by compulsory licensing.
- E-prescriptions will be introduced where the medicine name can be picked up from a drop-down menu of molecules.
- Doctors need not prescribe molecule names to patients
MAJOR CHALLENGES
The challenge for the government is to effectively equip themselves with both pre and post implementation procedures. They have to create an action plan for an easy transition and create an effective supply chain tracing and tracking mechanism. There should be proper portals and robust data procuring systems. The tremendous lobbying power of the pharmaceutical industry is another factor that can delay the implementation of this proposal. Besides, there are also concerns that retailers will use this situation to their advantage. Since brands will not be suggested by physician, power of discretion will now shift to retailers giving them an opportunity to recommend companies that offer the biggest margins. Margin pressures on companies can in turn tempt them to come up with poor quality medicines ultimately affecting consumers.
GAINERS AND LOSERS
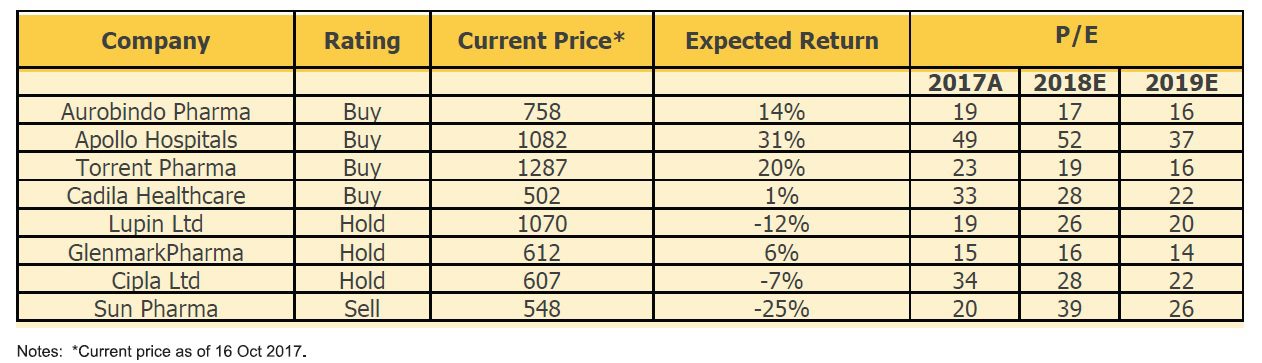
Domestic players with insufficient manufacturing capacities will be most affected. The current proposals will increase the cost to companies and the price capping will put pressure on upsides. This will in turn squeeze the profit margins. Smaller generic players are more likely to feel the greatest pressure. Large pharma companies may be able to manage such pressures as they will be in a position to manufacture in huge quantities. Besides, most of the large Indian pharmaceutical companies have a greater proportion of their sales to the West than to Indian markets. Some of the biggest generic players from India are Sun Pharmaceutical, Lupin Ltd, Dr Reddy Labs, Cipla and Aurobindo Pharma. Further, the ban on third party manufacturing will affect companies which outsource a large part of their productions.
CONSUMERS AND INDUSTRY WILL BENEFIT IN THE LONG-TERM
However, the objective of this new draft is to make the pharma industry more regulated, self-reliant, and fairly priced and quality focused through standardised practises which in itself is a very noble theme. The government is also conveying its intention to restructure the industry and resolve issues currently plaguing them on account of unreasonable trade margins and unfair practices employed by stockists, distributors and retailers. This will ultimately bring in more transparency and create a level playing field in the sector. Thus the final beneficiaries will be both the end consumers and the industry. It is therefore expected that if implemented effectively, the resultant pricing efficiency will reap benefits for the Indian pharmaceutical industry in the long term.
STOCKS UNDER COVERAGE